-
Posts
1,294 -
Joined
-
Last visited
Content Type
Profiles
Forums
Gallery
Posts posted by kareen
-
-
Mangroves do best under intense light. They are trees exposed to the same intense light found in all tropical reef zones. Mangroves can grow under less intense light, but they will thrive under metal halide lamps. I am growing my mangroves under 175 watt 10K metal halide lamps. I use 400 watt 14k metal halide lamps for my corals.
How do get mangroove propagate?
-
up for the day...
-
Morning,
I know I'm a little
 ..... but hor... if don't understand, simply dose is easy but fixing the problem later on is difficult... I would like to test out FeSO4 too....
..... but hor... if don't understand, simply dose is easy but fixing the problem later on is difficult... I would like to test out FeSO4 too....I am doing some reading up on this area to look for some answers.... Eg.
1) Red sea, pacific ocean, indo sea, etc... Is there a percentage differences in SO4 concentration?
The brown stuff are: Fe(OH)3, Brown solid locking away P- ions and some others.Left in solution are: NaSO4, MgSO4, CaSO4... something like that...
2) What is sodium sulphate, magnesium sulphate, calcium sulphate? Harmful? Or actually they are also part of the trace elements found in seawater....
I think direct dosing in tank will likely leave some trace of Fe(OH)3 in water or even left it in the fish gills.... sympton may not show up in short term.... Do the dosing external to tank is more secure.
A simple question hit my head....
3) What is actually rowaphos? Iron oxide? Can we buy the chemical in chemist just like FeSO4?
http://en.wikipedia.org/wiki/Ferric_oxide
rowaphos is going at about $75/kg from reefer pm reply.
4) What is the removal capacity of rowaphos versus Iron sulphate?
Appreciate any inputs if one knows.... [Thru' sharing, everyone learns more]
-
i remenber liao... are the 2 still there?

Well, I sold them off.... almost quit that time....
-
Hi nakazoru!
For every gram of FeSO4 dose, how many SO4 gets into the water?
chemical concentration ppm, mg/kg part of salinity %Chloride Cl 19345(ppm) 55.03%
Sodium Na 10752(ppm) 30.59%
Sulfate SO4 2701(ppm) 7.68%
Magnesium 1295(ppm) 3.68%
Calcium Ca 416(ppm) 1.18%
Potassium K 390(ppm) 1.11%
Bicarbonate HCO3 145(ppm) 0.41
From the ratio above, it will take about 350 ppm of SO4 to up another percentage point of a normal seawater ratio (7~8%)....
-
Hi nbc!
Correspond with CFOh. It's his unit....
-
Hi sis, think i met u once before.. or am i wrong
 yap, will be setting up the 5 footer, while my present tank be a School for FROZEN training classes.... i got most specis from Coral farm.. they have more selection there
yap, will be setting up the 5 footer, while my present tank be a School for FROZEN training classes.... i got most specis from Coral farm.. they have more selection there  Thanks for the tips! will follow your advice
Thanks for the tips! will follow your advice 
yup... we have met... I bought 2 giant squamosa from u.....
-
sps for newcomer ==> new tank ==> death sentence.
my 2 cents.
-
Do u intend to let the tank going seahorse-less while the plants establish? BTW, where u get so many species? From reefers?
Some pointers to prevent grape going asexual base on my experiences....
Choose a moderate to high flow area will prevent it going asexual. I am a little confident to speak so is due to recent event that has take place in my tank....
There is massive lailai dieing in the tank for two weeks and I believe the condition was pretty bad 'cos I lost an anthia.... It contacted pop eye disease.... During that two weeks, no asexual from the grape algae (about 1 feet away from Hydor K1) in the display tank where I clipped on to feed the fish, considering there is a period of total darkness in display daily...
-
UPS for probe and fish....
-
ups...
Also, friend got a red sea purple tang for sale as well....
-
I think most probably the infection and worms as I did not deworm the fella...
-
Kareen.
Oh, the PO4 test kit came with a sample PO4 solution, if no change in color, can throw away.
The drip? I do tonight.
-
Add a flat liverock to act as barrier between the two if possible....
-
My PO4 testkit use only 5 time then white elephant... now expired, cannot change color liao.
Could it be the PO4 is not detectable now? Do a little experiment to test out... Take some water from tank in a container, dump some food for it a day or two and then measure the PO4.....
PS: can explain what is Tay's drip?
PSS: oh ya, juz test water from the rotifer culture should have PO4....
-
I'm not sure if it's gd enuff for long term, but maybe u can help me judge:
- It's a bare 3ft tank (abt 200L) with a canister as the only filtration system (Currently only filter wool in the canister, but thinking of adding carbon)
- I also added 2 Seio pump (M620 & M1100) for water circulation..
- I'm keeping the lights on 24/7 cos they seem v stress everytime I off the light, probably cos no shelter (Gonna add a DIY shelter, currently waiting for the silicon to cure)
* I use those normal silicon, izzit safe for seawater?
Basically, that's it...

* Btw I have a weipro skimmer in my storeroom but not using cos of the heat generated by the Atman pump used to feed it..
Do u have any corals in the QT? If no, take away the Seio to reduce heat xfer to water.
What is the turnover for the canister? Make sure there is enough O2... Also regular clean up for canister 'cos it will choke over time.... I will prefer those cheap overhead kind of biology filter than canister due to easier maintainance.
As long as temperature not above 30, should be alright for the fish....
Like what colinsoon said, do not on lights for 24 hours.... and pipes for fish to hid....
-
I have not yet tried the FeSO4... 'cos I have a little spare time to do some water change over the weekend.... I have setup the plankton and rotifer though...
Where is the thread about Tay's drip method? I didn't know...
PO4 test kit veri expensive... do u think it's necessary to own one? To me, PO4 reading is not as useful as Ca or Mg.... Knowing the level will not enhance the reef... I will spend the money on saltmix, Ca testkit...
-
the green algae
looks more like turtle weeds than green hairline algae....
-
Btw since I still have some fish with me now in the QT, can the fish go in b4 the corals?
Well, how well equip is the QT? Is the tank able to provide the requirement for the long stay till your reef tank mature?
-
no lah...
The percentage I given is in mg/l, which is the absolute weight of the minerals, then different minerals has a different densityI think what you mean is that different minerals has different mass... and this make the cal different from mineral to mineral in different form... I understand... Thanks for explaination of mg/l and ppm....
-
oh ya.. the usual slant design make it more room space to carry out work on sump.
-
-
Morning Simon.
Sulfate SO4 2701(ppm) 7.68%Magnesium 1295(ppm) 3.68%
Calcium Ca 416(ppm) 1.18%
Looking at SO4 and Mg, SO4 is heavier than Mg but Mg has a higher ppm than SO4. Ca has lower density and ppm compare to the two mineralsFrom what u listed, is Mg higher ppm than SO4? I don't understand this part.... Also, how do we relate mg/l to ppm, the matrix we test on?

-



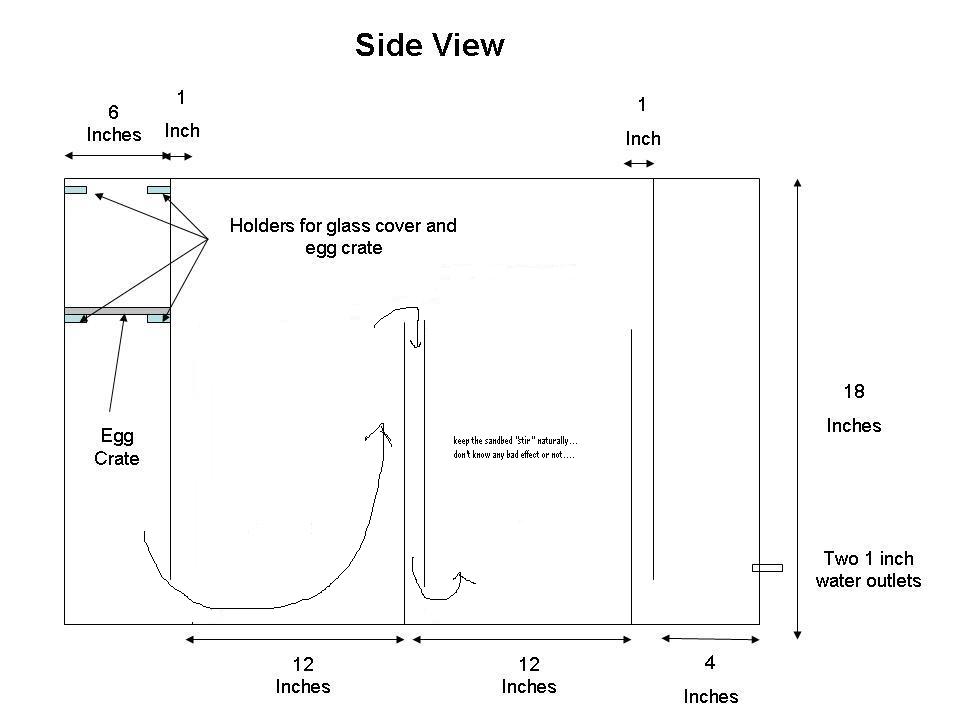
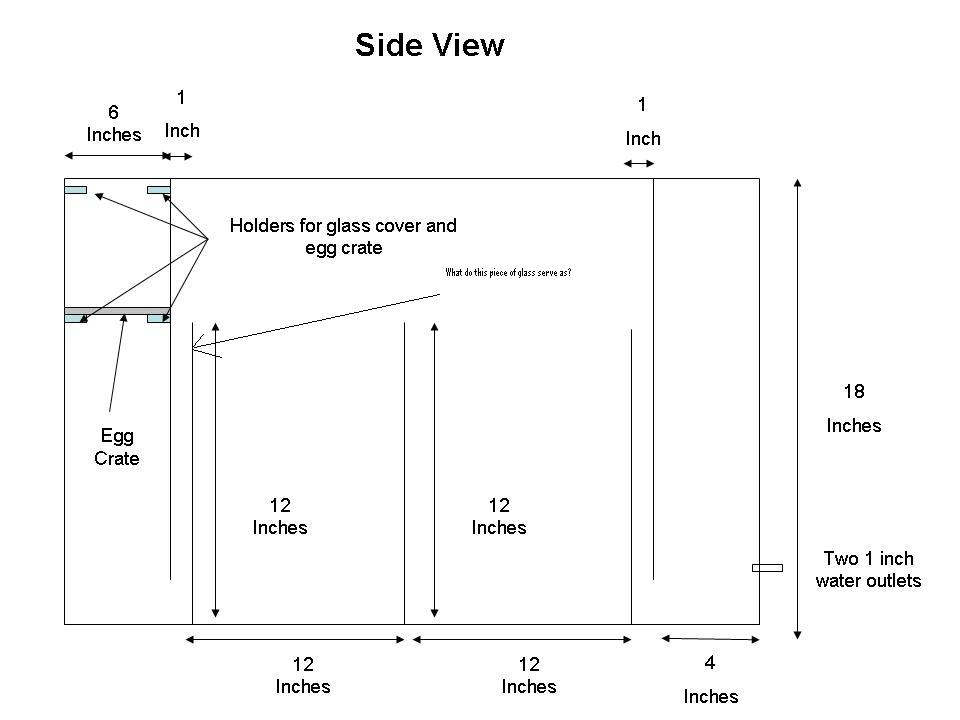
Sump Design
in DIY Forum
Posted
Well, this is my thought....
Why do u want to put skimmer outside? I will put it inside if I have a choice....
1) Better skimmer motor power when water is pump at ground 0 with little or no loss due to the elbow
2) no worry of leakage..
of course in sump at a location where simple maintainance easily....
Do u think there will be a sand storm at the sand where water is pass thru? For this, I'm not sure but I know energy is been 'eaten' as the distances goes on and on... and the sand will somehow being blow in a way where it is 'most stable' after a while....